Human Immunodeficiency Virus (HIV)
BASHH/BHIVA guidelines (see below for individual references).
HIV Investigation and Diagnosis Guidelines
Indications for HIV Testing
HIV testing is recommended in:
- People with features consistent with an HIV indicator condition
- People with HIV +ve sexual partners
- People attending the following services
- Sexual health
- Addiction and substance misuse
- Antenatal
- Termination of pregnancy
- Hepatitis B and C, TB and lymphoma
- People commencing chemotherapy or immunosuppressive or immunomodulatory therapy
- People belonging to groups at increased risk of exposure to HIV
- MSM
- Female sexual contacts of MSM
- Black Africans
- Current or prior injecting drug use
- Sex workers
- Prisoners
- Trans women
- From country with high diagnosed seroprevalence (>1%)
- Sexual contact with anyone from a country with high diagnosed seroprevalence (>1%)
- People accessing primary and secondary healthcare in areas of high and extremely high HIV seroprevalence (including emergency departments)
HIV Testing Diagnostic Pathway
Offer a 4th-generation combination assay (HIV p24 antigen + HIV antibody) with venous sampling as an initial screening test ASAP after potential exposure
- If +ve screening test → immediately perform a confirmatory assay on a different sample (ideally a fresh blood sample)
- Do not diagnose HIV based on 1 +ve test
- If -ve screening test → subsequent action depends on whether the testing is performed within the 45-day window period or not
- -ve test <45 days post-exposure → repeat test at ≥45 days
- -ve test ≥45 days post-exposure → no further test needed
If results are +ve from self-tests, confirmatory laboratory testing is required due to a small possibility of a false-positive result.
The 4th-generation combination assay has a window period of 45 days. The window period is the time between exposure and when a test can accurately detect it. Such that if the test is performed within the window period (<45 days post-exposure), there is a chance of false negative.
Baseline Post-Diagnosis Investigations
The following tests are recommended at baseline (organised into the following 3 categories):
| Category | Tests |
|---|---|
| HIV-related tests |
|
| Infection screen |
|
| Routine bloods |
|
References
HIV Management Guidelines – Antiretroviral Therapy (ART)
Indications and Timing
Choice of Drugs and Regimen
HIV is treated with antiretroviral therapy (ART), a treatment regimen typically comprised of 3 or more different antiretroviral drugs to overcome the risk of drug resistance.
HIV-1 (most cases in the UK)
1st line regimen
- Triple therapy (most commonly used) with 2 NRTIs and 1 integrase inhibitor
- Tenofovir AF (NRTI) + emtricitabine (NRTI) + bictegravir
- Tenofovir AF or DX (NRTI) + emtricitabine (NRTI) + dolutegravir
- Abacavir (NRTI) + lamivudine (NRTI) + dolutegravir
- Two-drug ART regimen
- Lamivudine (NRTI) + dolutegravir (integrase inhibitor)
- Strict criteria: no lamivudine resistance + RNA copies <500,000 + CD4 count >200 + no active hepatitis B
Pre-testing for HLA-B*57:01 is necessary before starting abacavir.
Tenofovir DX (disoproxil) is an older oral pro-drug, while tenofovir AF (alafenamide) is a newer oral pro-drug.
Tenofovir DX carries a higher risk of renal and bone toxicity than tenofovir AF.
HIV-2 (rare in the UK, endemic in West Africa)
HIV-2 has intrinsic resistance to all NNRTIs, therefore it is important to avoid them.
1st line: triple ART with 2 NRTI backbone and boosted protease inhibitor
- Tenofovir / abacavir (NRTI) + lamivudine (NRTI) + boosted darunavir (protease inhibitor)
Ongoing Follow-up and Monitoring
- Minimum yearly review
- Aim for viral load of <50 copies/mL
References
HIV PrEP (pre-exposure prophylaxis)
HIV Testing in PrEP
3-monthly HIV testing should be routinely offered as part of monitoring for PrEP.
Indications
Main indications are:
-
Condomless receptive anal sex (MSM and trans-women)
-
Condomless sex with HIV +ve partner with VL >200 copies/mL
-
IVDU with shared equipment (not routinely recommended if effective needle-exchange or opioid substitution programmes exist)
Drugs and Regimen
1st line regimen (dual NRTI): tenofovir disoproxil + emtricitabine (300/200 fixed dose combo)
There are 2 main dosing regimens:
| Dosing regimen | Suitability | Description |
|---|---|---|
| Daily PrEP | All high-risk individuals | One tablet daily → until 7 days after last exposure |
| On-demand PrEP | Anal exposures ONLY | 2+1+1 (2 tablets pre-sex → 1 tablet 24 hr post sex → 1 tablet 48 hr post sex)
If multiple-day exposure → 1 tablet daily until 48 hr after last exposure |
It is important to ensure the person is HIV -ve before taking PrEP. As PrEP regimens contain only 2 drugs, if the patient is already HIV +ve, using only 2 agents risks sub-therapeutic suppression (allowing the virus to replicate) and drug resistance.
Reference
HIV PEP (post-exposure prophylaxis)
Indications
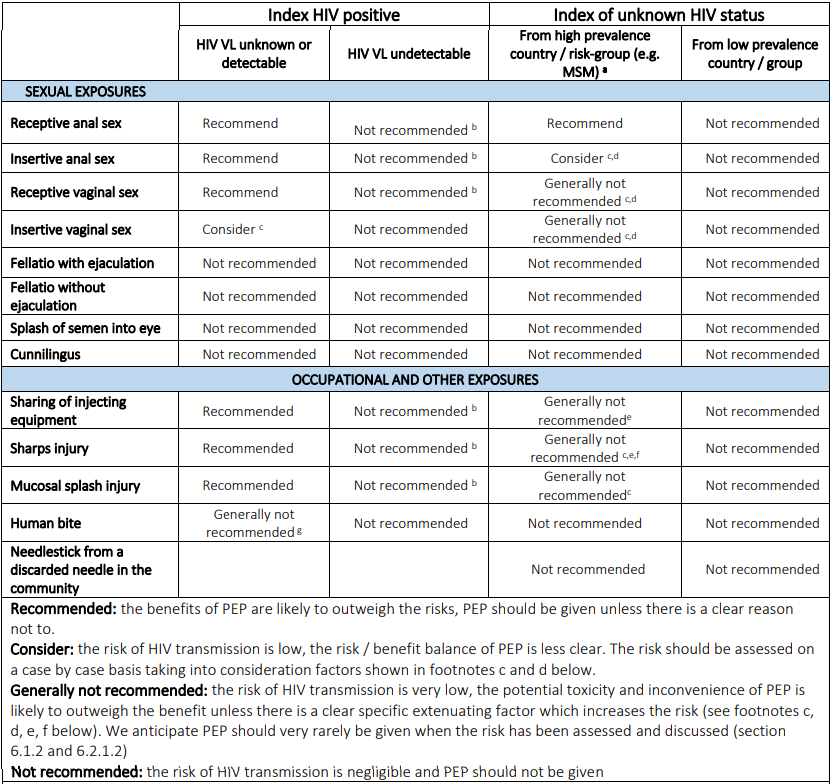
Indications for PEP are categorised and presented below:
| Type of exposure | Indications |
|---|---|
| Sexual exposure | If index HIV +ve
If index HIV status is unknown
|
| Occupational exposure | Only indicated if index HIV +ve AND any of the following:
|
Key points about the risk of transmission:
- Receptive sex carries a higher risk than insertive sex.
- Anal sex carries a higher risk than vaginal sex.
BASHH/BHIVA – Summary table of PEP prescribing recommendations
Baseline Tests
Baseline tests required before initiating PEP:
- HIV-1 Ag/Ab
- Serum creatinine and eGFR
- Alanine transaminase
- Hepatitis B serology – if not vaccinated or with documented HepBsAb >10 IU
- Chlamydia, gonorrhoea, syphilis testing – if from sexual exposure
- Hepatitis C screening – if from occupational exposure OR those at risk from sexual exposure (e.g. MSM)
Drugs and Regimen
1st line regimen: 3 drug tenofovir (NRTI) + emtricitabine (NRTI) + raltegravir (integrase inhibitor)
Timings:
- Start PEP within 72 hours (ideally <24 hours, guidelines say not to initiate PEP if >72 hours)
- Duration of PEP: 28 days (4 weeks)
Follow Up
Repeat HIV testing at 45 days after completion of the 28‑day PEP course (not 45 days after exposure).
Reference
HIV in Pregnancy
See the HIV and Pregnancy article.